Healthcare Software Development: The Ultimate 2025 Guide

Julia Teryokhina, Senior Presales Engineering Manager
April 18, 2025
The union of IT and healthcare has brought medicine to a new qualitative level, and in 2025, healthcare software development is more critical than ever.
Medical services have become more accessible: you no longer need a face-to-face appointment with a doctor; instead, you can use telemedicine software to get advice online or leverage EHR systems for seamless patient data management. With diagnostic tools from the Internet of Medical Things (IoMT) subset, you can perform remote health monitoring. The past pandemic underscored the importance of prioritizing well-being, and the Zoomer generation continues to advocate for a health-conscious, eco-friendly lifestyle. These trends underscore the increasing demand for innovative solutions, such as AI-driven diagnostics and HIPAA-compliant software, in healthcare software development.
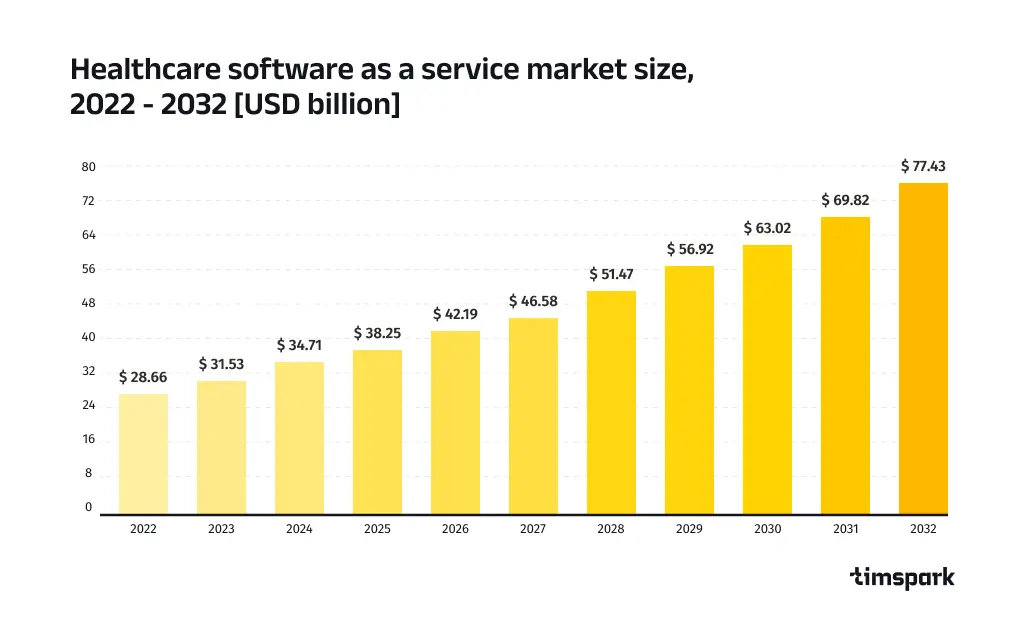
According to Precedence Research, the global healthcare software development market was valued at $28.66 billion in 2022 and is expected to reach approximately $77.43 billion by 2032. Ready to launch your own healthcare software development project in 2025? This guide covers everything you need to know, from compliance to cutting-edge trends.
Challenges in healthcare software development: compliance and beyond
Before you start healthcare software development in 2025, it’s crucial to understand the unique challenges involved. Developing solutions like EHR systems or telemedicine apps often requires mandatory certification to ensure user safety. Skipping critical steps can lead to reclassification as a Wellness application—or worse, the need for new investments to address issues and pursue the coveted CE mark. Beyond financial investment, time to market remains a key concern, especially with rising competition in the healthcare tech space.
Public data reveals that 75% to 98% of healthcare startups fail, often due to certification hurdles that delay software delivery to end users. Additional challenges, such as cybersecurity risks and scalability demands, further complicate the process, particularly for AI-driven or IoMT-based solutions.
Certification hurdles in healthcare software development
Time to market and financial risks in healthcare software development
Cybersecurity and scalability challenges in healthcare software development for 2025
Scalability is equally critical; an EHR system must handle 10,000 daily users, while genomics analytics platforms process 500TB of data annually, demanding elastic cloud infrastructure like AWS or Azure. A 2024 Gartner report notes that 45% of healthcare apps fail to scale due to poor architecture, leading to downtime and user churn. Addressing these challenges requires integrating cybersecurity from the design phase and adopting scalable architectures—microservices can reduce downtime by 30%, per a 2024 study.
The Gold Standard: IEC 62304 for healthcare software compliance
Safety classification for healthcare software: A, B, C risks
These classifications guide developers in meeting compliance requirements:
- Class A: The software poses no risk of injury, such as an EHR system for data storage.
- Class B: The software may cause minor injury, like a telemedicine app with basic diagnostic features.
- Class C: The software can cause serious injury or death, such as AI-driven surgical tools requiring rigorous oversight.
Proper classification ensures that healthcare software development aligns with safety standards, minimizing risks and delays. Learn more in the IEC 62304 safety classification guidelines or explore certification details .
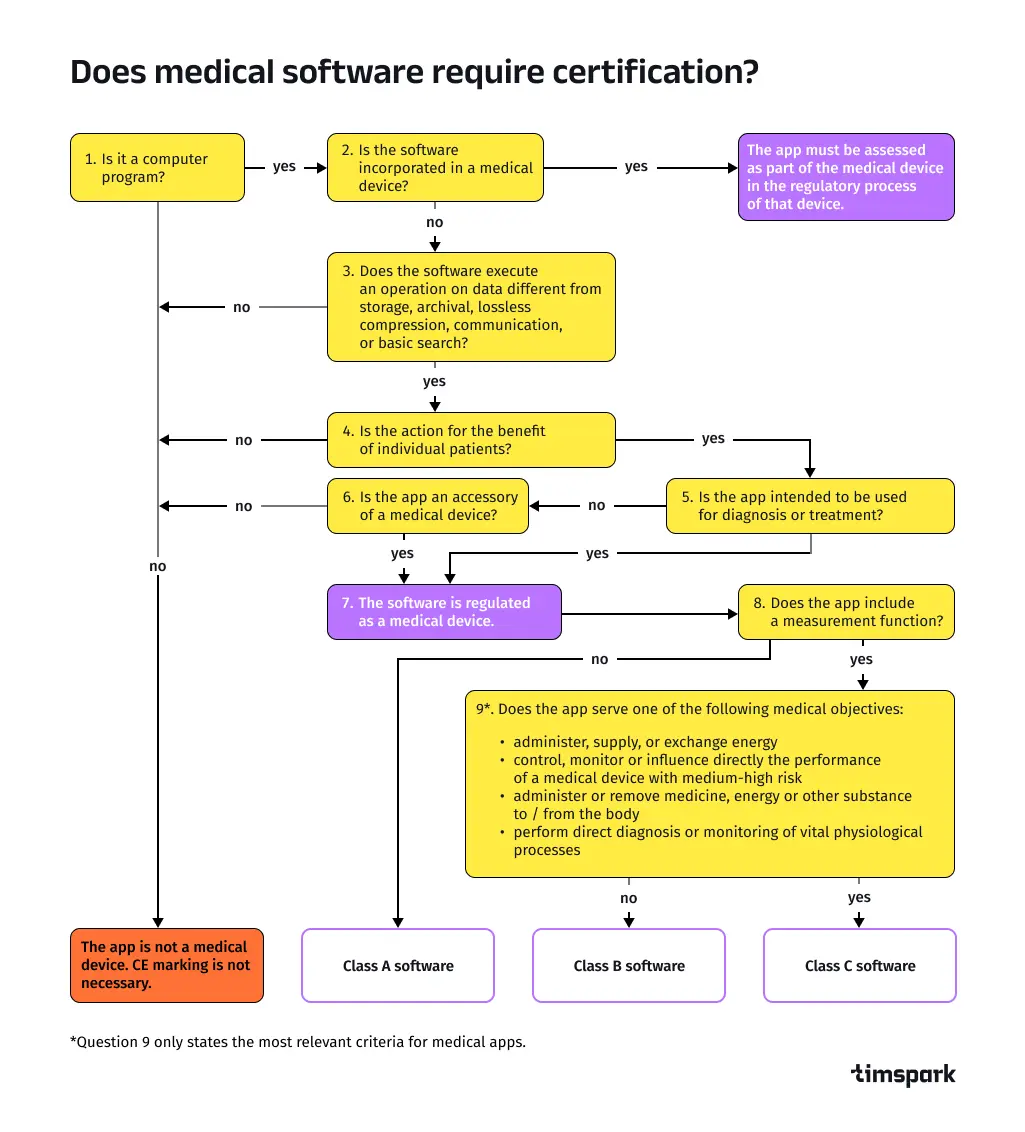
Does healthcare software require certification?
The certification process in healthcare software development varies by safety class under the IEC 62304 standard International Electrotechnical Commission:
- Class A: Software like EHR systems for data storage can be self-certified. Developers declare adherence to IEC 62304 and publish a control document outlining compliance.
- Class B: Software such as telemedicine apps with diagnostic features requires a notified body to verify IEC 62304 compliance, ensuring safety protocols are met.
- Class C: High-risk software, like AI-driven surgical tools, undergoes rigorous scrutiny—notified bodies analyze development processes, design documentation, and the software itself.A CE mark indicates the software has passed assessments and is safe for use in 2025, as required by the EU MDR for European markets. For detailed compliance steps, refer to the .
Essential documentation for healthcare software compliance
Document | Class A | Class B | Class C |
Software development planning | yes | yes | yes |
Software requirements analysis | yes | yes | yes |
Software architectural design | yes | yes | |
Software detailed design | yes | ||
Software unit implementation | yes | yes | yes |
Software unit verification | yes | yes | |
Software integration and integration testing | yes | yes | |
Software system testing | yes | yes | yes |
Software release | yes | yes | yes |
Local regulations for healthcare software: HIPAA, GDPR, and more
SOUP and OTS components in healthcare software development
Testing specifics for healthcare software solutions
Hosting challenges for healthcare software in 2025
Winning 12-step strategy for healthcare software development in 2025
Step 1: Define your healthcare software’s MVP and integrations
Step 2: Research your healthcare software’s target audience
Step 3: Build a collaborative UI prototype for healthcare software
Step 4: Analyze requirements for healthcare software compliance
Step 5: Design a modern architecture for healthcare software
Step 6: Plan agile milestones for healthcare software development
Step 7: Develop riskiest features first in healthcare software
Step 8: Test with real users using modern methods in healthcare software
Step 9: Ensure security and load testing for healthcare software
Step 10: Use cloud-based environments for healthcare software testing
Step 11: Certify your healthcare software with updated standards
Step 12: Launch and monitor healthcare software with AI-driven insights
Launch your healthcare software in 2025 with a phased rollout, starting with a pilot for an EHR system or a regional release for a telemedicine app. Utilize AI-driven monitoring tools, such as Dynatrace, to track performance—40% of healthcare apps experience post-launch issues, according to a 2024 study by Gartner. Gather feedback with Typeform and implement continuous improvements, ensuring long-term user satisfaction and compliance with evolving regulations.
Why choose Timspark for your healthcare project?
Timspark is your trusted partner. With over 1,000 engineers across offices in the UK, Poland, Georgia, and Bulgaria, we bring unparalleled expertise to deliver HIPAA-compliant and GDPR-compliant solutions.
Our Core Teams—specialists in AI, cybersecurity, and telehealth—have powered platforms with 1.5M daily users, reducing time-to-market by 30%. We prioritize security, using OWASP and NIST 800-115 penetration testing to protect sensitive data, addressing the 18.1% annual growth in healthcare cybersecurity needs (2022-2028). From staffing your team with top talent to managing your entire project, we optimize costs and guide you through the development lifecycle, ensuring compliance and innovation.
Contact us and our leading experts will help you optimize costs and guide you through the entire software development lifecycle.
Our projects in Healthcare industry
Looking for a healthcare software development company?
References
- Healthcare Software As A Service Market. Precedence Research, 2022.
- What Leads to Company Underperformance & Failure? TTi Health Research & Economics, 2021.
- Startup Failure and Success Rates: 2023 Research. StartupTalky, 2023.
- IEC 62304:2006. Medical device software. ISO, 2021.