Clinical Trial Data Management Platform: Modernized System and Enhanced Performance
Clinical Trial Data Management Platform: Modernized System and Enhanced Performance
Timspark migrated a clinical data management system populated with health and research records from the cloud to a new code-based platform. We revamped the platform’s architecture and handled ongoing support for the solution.
#DataManagement
Client*
The client is a global leader in developing pioneering healthtech solutions focused on driving innovative data management in clinical trials and the industry as a whole.
*We cannot provide any information about the client or specifics of the case study due to non-disclosure agreement (NDA) restrictions.
Project in numbers
The team involved in the project
Challenge
The main goal was to upgrade the outdated platform, turning the traditional e-data fetching solution into advanced software for clinical trial data management. Additionally, the client aimed to bolster the clinical data management software’s security capabilities by introducing HIPAA and GDPR compliance features.
Related objectives
Solution & functionality
Data collection
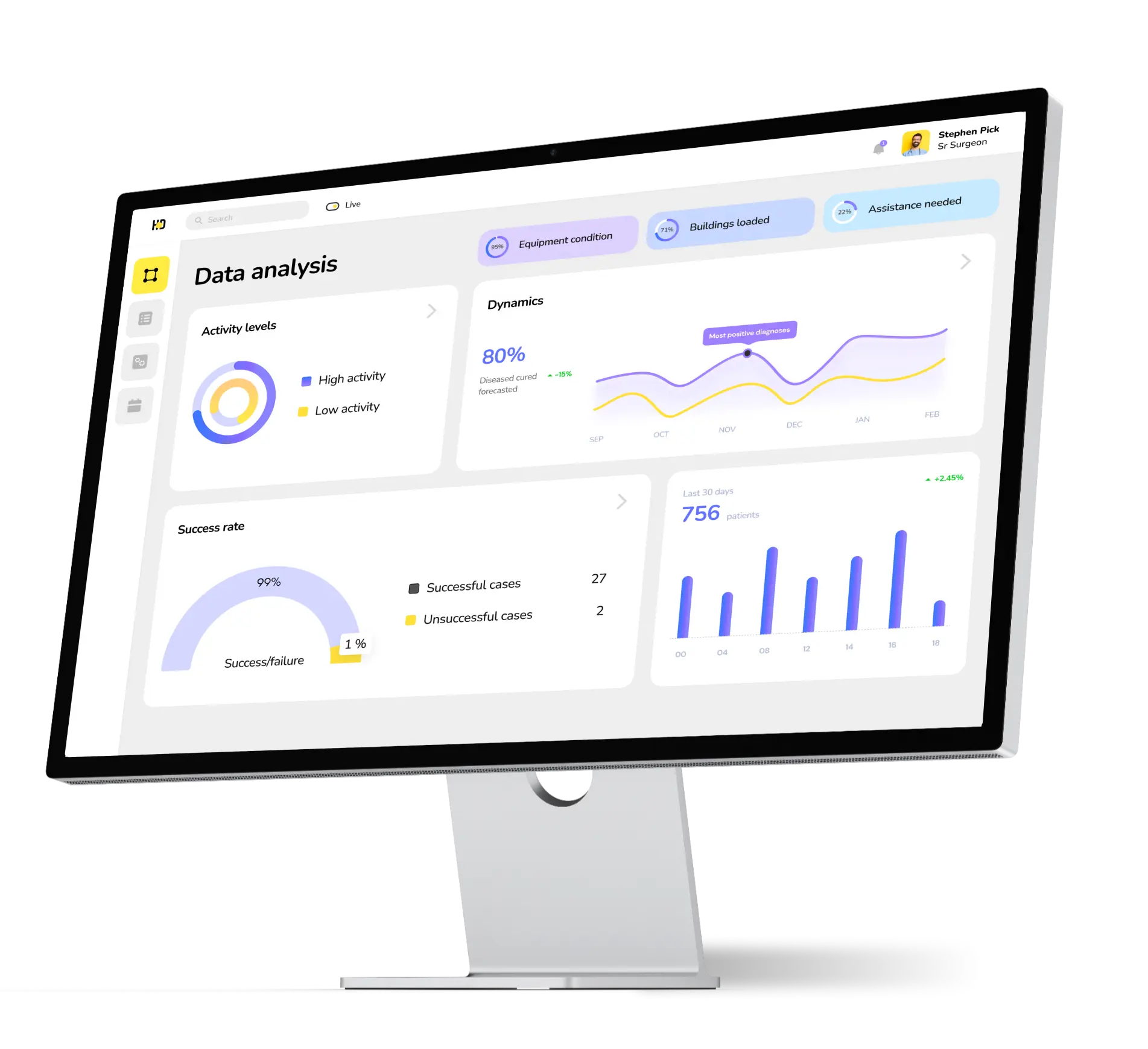
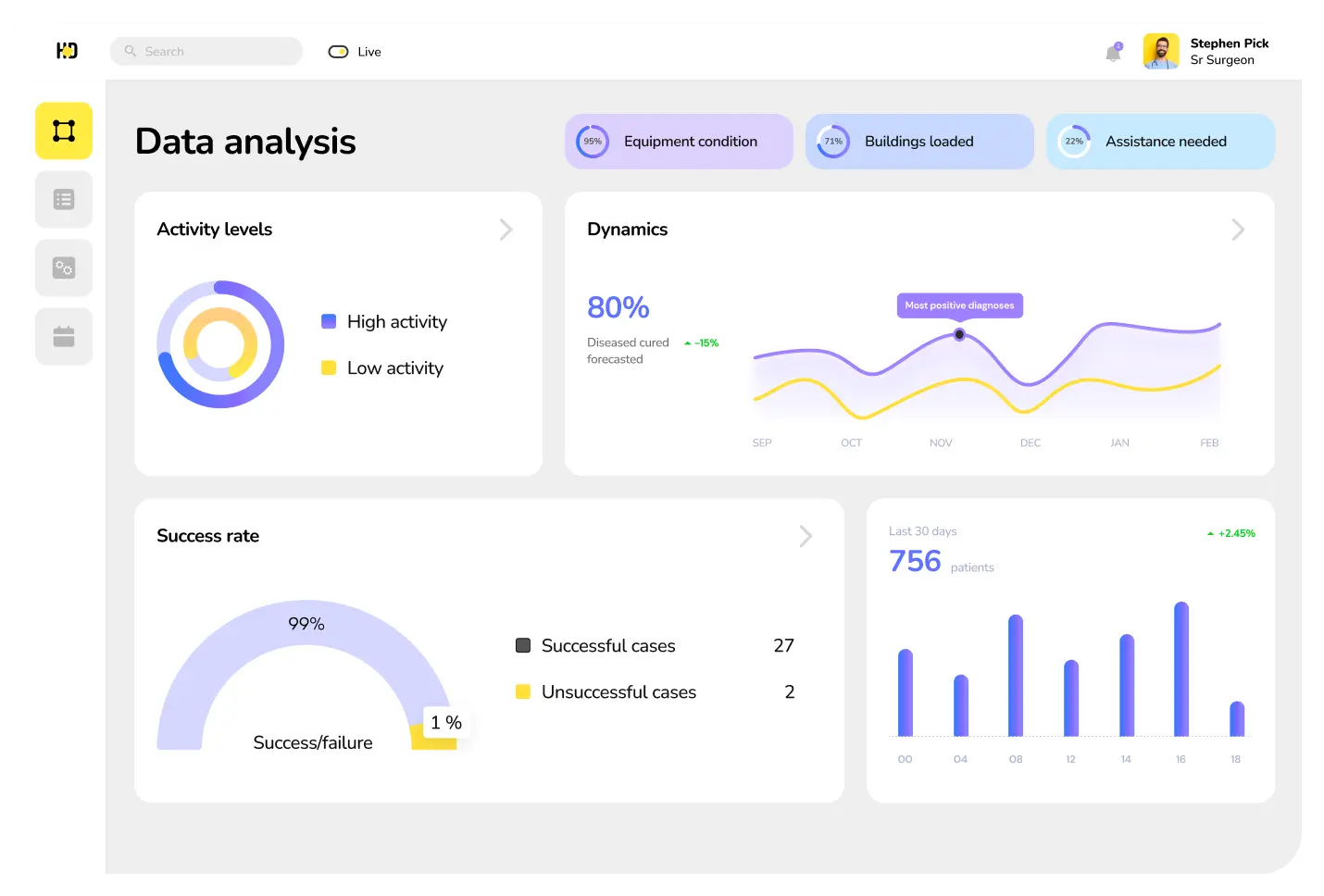
The primary function of the clinical data management system is to gather data concerning clinical trials for subsequent integration, management, analysis, and finding correlations. This solution supports the entire research process cycle and offers users advanced reporting capabilities.
Data management
The data-centric software for clinical trials is tailored for diverse medical organizations, aiming to digitize their internal processes. These organizations encompass medical labs, medical device manufacturers, pharmaceutical and biotech institutions, and contract research organizations (CROs).
Results and business value
Higher performance
We’ve enhanced the system’s performance, stability, and usability, elevating its appeal for data management in clinical trials across the market.
Long-term vision
Given the resounding success during our collaboration in the active project phase, our IT specialists seamlessly transitioned to providing long-term support for the solution.
Benefits for client
The client highlighted the exceptional skills of our full-stack developers. The company has finally found a partner that is both technically and regulatory savvy, as well as a good communicator.
Related cases
Need assistance with a software project?
Whether you're looking for expert developers or a full-service development solution, we're here to help. Get in touch!
What happens next?
An expert contacts you after thoroughly reviewing your requirements.
If necessary, we provide you with a Non-Disclosure Agreement (NDA) and initiate the Discovery phase, ensuring maximum confidentiality and alignment on project objectives.
We provide a project proposal, including estimates, scope analysis, CVs, and more.
Meet our experts!
Viktoryia Markevich
Relationship manager
Samuel Krendel
Head of partnerships